

1. Hypochlorous acid is a free chlorine molecule with the chemical structure HOCl. It is the dominate free chlorine species in chlorine solutions that have a slightly acidic to neutral pH. HOCl is a much more powerful oxidant than sodium hypochlorite (or chlorine bleach).
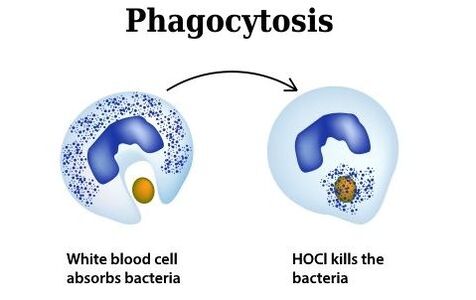
2. Hypochlorous acid is produced naturally by the white blood cells of all mammals. It is used by white blood cells to kill invading microbial pathogens
3. Hypochlorous acid (HOCl) is a neutrally charged molecule. Bacteria have negatively charged cell walls. Just like magnets, molecules with the same charge will repel each other. For example, the negatively charged molecule of bleach (OCl-) is repelled by bacterial cell walls. This is not the case with HOCl which is neutrally charged. HOCl easily penetrates bacterial cell walls. HOCl either oxidizes the cell walls killing the bacteria or enters through the cell walls and destroys the vital components inside the bacteria.
4. Chlorine is an extremely effective disinfectant for inactivating bacteria. A study conducted during the 1940s investigated the inactivation levels as a function of time for E. coli, Pseudomonas aeruginosa, Salmonella typhi, and Shigella dysenteriae (Butterfield et al., 1943). Study results indicated that HOCl is more effective than OCl- (aka. chlorine bleach) for inactivation of these bacteria. These results have been confirmed by several researchers that concluded that HOCl is 70 to 80 times more effective than OCl- for inactivating bacteria (Culp/Wesner/Culp, 1986). Since 1986, there have been hundreds of publications confirming the superiority of HOCl over OCl- (click here to visit research database). HOCl may be more effective than OCl- for two reasons, this first is because it holds a neutral charge and therefore can easily penetrate the negatively charged cell walls of bacteria. The second reason is because HOCl has a much higher oxidation potential than OCl-.
5. Hypochlorous is a powerful oxidant and is 100 times more efficient at killing microbial pathogens than sodium hypochlorite (aka. chlorine bleach). Hypochlorite is very unstable, but hypochlorous acid is stable and is highly microbicidal, active against bacteria, viruses, and fungi.
Physiology
Hypochlorous acid is one of the most effective known biocides.The chemical structure is HOCl. It is produced by the human immune system to kill invasive organisms and fight infection. White blood cells in the human immune system produce hypochlorous acid through the myeloperoxidase-mediated peroxidation of chloride ions. White blood cells release this natural oxidant to fight invading pathogens. Almost 99% of the mass of the human body is made up of six elements: oxygen, carbon, hydrogen, nitrogen, calcium, and phosphorus. Only about 0.85% is composed of another five elements: potassium, sulfur, sodium, chlorine, and magnesium. All 11 are necessary for life.
Why is pH important?
The pH dictates the free chlorine species present in aqueous solutions. At a pH of between 5-6, the chlorine species is nearly 100% hypochlorous acid (HOCl). As the pH drops below 5, it starts to convert to Cl2 (chlorine gas). Above a pH of 6, it starts to convert to the hypochlorite ion (OCl-).
There are three forms of free available chlorine: chlorine gas, hypochlorous acid and hypochlorite. Assuming a constant temperature of 25 degrees Celsius, when the pH is below 3, free chlorine will leave solution as chlorine gas. When the pH is above 7.5, over 50% will be hypochorite (OCl-) and will increase in hypochlorite as it rises toward pH 14. Between pH 3 and pH 7.5 the free chlorine solution will be dominated by hypochlorous acid (HOCl). In other words in acid conditions the solution produced will have a high hypochlorous acid concentration, but will also contain dissolved gaseous chlorine, which can be corrosive, at a neutral pH the solution will be around 75% hypochlorous acid and 25% hypochlorite.
Hypochlorous acid is a weak acid (pKa of about 7.5), meaning it dissociates slightly into hydrogen and hypochlorite ions as noted in equation: : HOCl ⇌ H+ + OCl-
Between a pH of 6.5 and 8.5 this dissociation is incomplete and both HOCl and OCl- species are present to some extent. Below a pH of 6.5, no dissociation of HOCl occurs, while above a pH of 8.5, complete dissociation to OCl- occurs.
As the germicidal effects of HOCl is much higher than that of OCl-, chlorination at a lower pH is preferred. The germicidal efficiency of hypochlorous acid (HOCl) is much higher than that of the hypochlorite ion (OCl-). The distribution of chlorine species between HOCl and OCl- is determined by pH, as discussed above.
Because HOCl dominates at low pH, chlorination provides more effective disinfection at low pH. At high pH, OCl- dominates, which causes a decrease in disinfection efficiency.
Bacteria Inactivation
Chlorine is an extremely effective disinfectant for inactivating bacteria. A study conducted during the 1940s investigated the inactivation levels as a function of time for E. coli, Pseudomonas aeruginosa, Salmonella typhi, and Shigella dysenteriae (Butterfield et al., 1943). Study results indicated that HOCl is more effective than OCl- for inactivation of these bacteria. These results have been confirmed by several researchers that concluded that HOCl is 70 to 80 times more effective than OCl- for inactivating bacteria (Culp/Wesner/Culp, 1986). Since 1986, there have been hundreds of publications confirming the superiority of HOCl over OCl- (visit research database ).
This biggest challenge has been to create hypochlorous acid at a near neutral pH instead of chlorine gas or hypochlorite, and to do so in a stable form. Hypochlorous acid is a meta-stable molecule. It wants to revert back to salt water or convert to hypochlorite.
Hypochlorous acid safety
Multiple studies have reported on the safety profile of HOCl. The FDA has declared HOCl as G.R.A.S., which is Generally Recognized as Safe. HOCl is tasteless and odorless and poses no risk of irritation or toxicity.
Hypochlorous acid chemical formula HOCl;
Molar mass 52.46 g/mol;
Appearance Colorless aqueous solution;
Density Variable;
Solubility in water Soluble;
Acidity (pKa)7.53; and Conjugate base Hypochlorite.
Regulatory Body
EU-EPA
USDA
USA-FDA
Japan: Ministry of Health, Labor and Welfare
FDA-FSIS
Korea-FDA
Rating
Approved as “Sterilizer Producing Equipment”
Approved as Sterilizer for E. Coli 0-157, Salmonella and various other disease-forming bacteria
GRAS (Generally Recognized as Safe)
Approved to be used to wash fruits, vegetables and seafood
Food Sterilizer and Sanitizer
Sterilizer and sanitizer of slaughtered poultry, cattle and swine
-Food additive
- Sterilizing agent
Date
98
1999
Issue Number 00-03-13: 2000
Jun-02
2006
Nov-07
Aug-08
Summary of United States Regulation - Hypochlorous Acid
FDA Food Contact Notification 1811 - Hypochlorous Acid at up to 60 ppm for Produce, Fish & Seafood, Meat and Poultry Sanitation Hypochlorous acid may may be used in processing facilities at up to 60 ppm for use in process water or ice which comes into contact with food as a spray, wash, rinse, dip, chiller water, and scalding water for whole or cut meat and poultry, including carcasses, parts, trim, and organs; in process water, ice, or brine used for washing, rinsing, or cooling of processed and pre-formed meat and poultry products as defined in 21 CFR 170.3(n)(29) and 21 CFR 170.3(n)(34), respectively; in process water or ice for washing, rinsing or cooling fruits, vegetables, whole or cut fish and seafood; and in process water for washing or rinsing shell eggs. Visit Source at FDA Website
FDA Guidance for Industry: Guide to Minimize Microbial Food Safety Hazards of Fresh-cut Fruits and Vegetables The antimicrobial activity of a chlorine-based disinfectant depends on the amount of hypochlorous acid (also called "free chlorine") present in the water. The amount of hypochlorous acid in the water depends upon the pH of the water, the amount of organic material in the water, and, to some extent, the temperature of the water. If the amount of hypochlorous acid is not maintained when the amount of organic material increases, the antimicrobial agent may lose effectiveness in maintaining water quality. If a fresh-cut processor uses a chlorine containing compound as a disinfectant, we recommend that the processor monitor the processing water for free chlorine or hypochlorous acid concentrations. Visit Source at FDA Website
EPA: Food-Contact Surface Sanitizing Solutions - Allowance of Hypochlorous Acid at up to 200 ppm The following chemical substances when used as ingredients in an antimicrobial pesticide formulation may be applied to food-contact surfaces in public eating places, dairy-processing equipment, and food-processing equipment and utensils. When ready for use, the end-use concentration of all hypochlorous acid chemicals in the solution is not to exceed 200 ppm determined as total available chlorine. Visit Source at EPA Website
FDA
USDA
EPA
How Does Bleach Work?
How the popular household cleaning staple removes stains and more.
By
Anne Marie Helmenstine, Ph.D.
Updated September 03, 2019
Bleach is a chemical that can remove or lighten color, usually via oxidation.
Types of Bleach
There are several different types of bleach:
Other bleaching agents include sodium persulfate, sodium perphosphate, sodium persilicate, their ammonium, potassium, and lithium analogs, calcium peroxide, zinc peroxide, sodium peroxide, carbamide peroxide, chlorine dioxide, bromate, and organic peroxides (such as benzoyl peroxide).
While most bleaches are oxidizing agents, you can use other processes to remove color. For example, sodium dithionite is a powerful reducing agent that you can use as a bleach.
How Bleach Chemicals Work
An oxidizing bleach works by breaking the chemical bonds of a chromophore (part of a molecule that has color). This changes the molecule so that it either has no color or reflects color outside the visible spectrum.
A reducing bleach works by changing the double bonds of a chromophore into single bonds. This alters the optical properties of the molecule, making it colorless.
In addition to chemicals, energy can disrupt chemical bonds to bleach out color. For example, the high energy photons in sunlight (such as ultraviolet rays) can disrupt the bonds in chromophores to decolorize them.
Cite this Article
Product Description
1. There are no better products for your home, work, or anywhere you go. Use as often as desired because daily cleansing will proactively create a safe and healthy environment. No gloves necessary! No rinsing necessary!
2. There are many uses for hypochlorous products, but each product has specific advantages that make it unique. Surface Cleanser comes in a convenient 8oz bottle so it’s easy to take everywhere! Several hypochlorous products come in 2oz spray bottles which are ideal for travel on airplanes and great for personal use to maintain personal hygiene or to keep your environment clean throughout the year. Be sure to look for more information under Testimonials on our website and to follow us on social media for up to date information and use cases.
3. Hypochlorous Acid (HOCl), sometimes known as anolyte water is a liquid that was first used over 175 years ago! It is produced by electrolyzing salt water in an anode chamber of an electrolyte cell. An electro –chemical reaction takes place and it converts an aqueous solution of Sodium Chloride (salt water) into a solution known as Anolyte, or electrolyzed water. The solution at that point contains >99.3% water, with the remaining solution being chloride salts and hypochlorous acid. Hypochlorous Acid is a weak acid (similar pH to intact skin), is a naturally occurring substance in our bodies and the same chemical that our own white blood cells produce to fight infection and kill bacteria. The overwhelming impediment to its widespread use has been shelf stability. As a Disinfectant Hypochlorous Acid solutions* are lethal to all known dangerous bacteria and viruses that threaten our health. *Note: See specific kill claims for hypochlorous products.
4. Hypochlorous products are very different than bleach and are not made from bleach. The active ingredient in bleach is 5% to 6% sodium hypochlorite resulting in an average pH of 12 which is highly alkaline. The main ingredient, hypochlorous acid, is a different oxychlorine species that is dissimilar from sodium hypochlorite (only a minute trace results naturally from the process of electrifying a proprietary salt solution). The pH of hypochlorous products is in a neutral range just like the pH of the dermal layer of all humans. Bleach solutions, or “Dakins Solution”, are of a very high pH, many of which are not shelf stable, and have to be used quickly.
5. The PH Level of the Hypochlorous First Aid Solution is in the range from 6.1 to 6.8.
Uses of Hypochlorous Acid
Hypochlorous acid has been investigated as a possible wound care agent, and as of early 2016 the U.S. Food and Drug Administration has approved products whose main active ingredient is hypochlorous acid for use in treating wounds and various infections in humans and pets. It is also FDA-approved as a preservative for saline solutions.
In a recent study, a saline hygiene solution preserved with pure hypochlorous acid was shown to reduce the bacterial load significantly without altering the diversity of bacterial species on the eyelids. After 20 minutes of treatment, there was >99% reduction of the Staphylococci bacteria.
Chemical formula HOCl; Molar mass 52.46 g/mol; Appearance Colorless aqueous solution; Density Variable; Solubility in water Soluble; Acidity (pKa)7.53; and
Conjugate base Hypochlorite
Hypochlorous Acid Effectiveness or Product Information
A bacterium is engulfed by immune system cells in an animated frame from The Secret Life of Bleach video.
By Joan B. Rose, PhD
March 23, 2018
We live in an age in which scientists regularly reveal remarkable details of the inner workings of the human body. Recently, a group of German researchers shed new light on the composition of the “antibacterial cocktail” that our immune systems concoct to fight off infection.1 The scientists demonstrated that the active chemical in that cocktail is none other than hypochlorous acid, the active ingredient in chlorine bleach.
First Responders in the Body
A bacterium is engulfed by immune system cells in an animated frame from The Secret Life of Bleach video.Phagocytosis is the term used to describe the ingestion of a cell or cell fragment. Immune system cells in the human body are the first responders to bacterial infection, initially surrounding the pathogenic cells with a strong potion that includes hydrogen peroxide and hypochlorous acid, and then ingesting the resulting “broth.” The soaking destroys invader bacteria by chemically oxidizing them. Until the new study was published, however, it was unclear which of the components of the “cocktail” is most important to phagocytosis.
Help from Genetically Engineered Cells
The researchers used genetically engineered E. coli to track the process of bacterial destruction by oxidation. These bacteria contain fluorescent proteins that normally glow under blue light, but, when oxidized, also glow under violet light. Experiments showed glowing under violet light begins in seconds but only after the start of phagocytosis, indicating the almost immediate release of the chemical cocktail following the surrounding of bacteria by immune cells.
Two enzymes in the immune cells are critical to the production of hydrogen peroxide and hypochlorous acid for the chemical cocktail. First, enzyme #1, when activated, produces a compound that is transformed into hydrogen peroxide. Next, enzyme #2 uses that hydrogen peroxide to produce hypochlorous acid. The scientists found that cells without a working enzyme #1 (unable to produce hydrogen peroxide, and therefore, hypochlorous acid) could not oxidize invasive bacteria. Cells lacking enzyme #2 (able to produce only hydrogen peroxide) could oxidize bacteria, but not efficiently. The team used logic to deduce that hypochlorous acid is the more active ingredient in the chemical mix.
Nothing New under the Sun?
Hundreds of thousands of years before chlorine bleach was invented and used by people for laundry whitening and disinfection, the human body was producing and utilizing hypochlorous acid internally to ward off infection for millennia. This point is made clear in The Secret Life of Bleach video featuring the work of University of Michigan professor and researcher, Dr. Ursula Jakob. Dr. Jakob and her team revealed the mechanism by which bleach destroys proteins in bacteria by “unfolding” their complex, three-dimensional structures. This insight, combined with the new research, presents an even more detailed perspective on how our bodies use hypochlorous acid, a chemical product identical to the active ingredient in chlorine bleach, to fight infection and stay well.
Joan B. Rose, PhD, is the Homer Nowlin Chair in Water Research at Michigan State University, the editor of the Global Water Pathogen Project, and a member of the Water Quality & Health Council. In 2016, Dr. Rose was named the Stockholm Water Prize Laureate for her tireless contributions to global public health.
Click here to download this article.
1Degrossoli, A. et al., “Neutrophil-generated HOCl leads to non-specific thiol oxidation in phagocytized bacteria,” eLife 2018;7:e32288 DOI: 10.7554/eLife.32288. On line, available: https://elifesciences.org/articles/32288
Exploring the mechanism of hypochlorous acid decomposition in aqueous solutions
Abstract
Hypochlorous acid is an intermediate in important industrial processes such as the production of chlorate but is also used for water treatment and disinfection. In aqueous solutions hypochlorous acid may decompose into oxygen or chlorate. Using density functional theory (DFT) modelling we have for the first time established detailed mechanisms for the respective decomposition pathways. Our calculations indicate, that both oxygen and chlorate formation proceed through an identical set of intermediates. At neutral pH the reaction is initiated by a fast equilibrium between HOCl, OCl−, Cl2O and Cl3O2−. The subsequent abstraction of Cl− to form Cl2O2 is rate determining for chlorate formation while it is the decomposition of Cl2O2 in the case of oxygen formation. Under alkaline conditions, OCl− decomposition to chlorate proceeds through chlorite. This reaction path is significantly less active. The highest rate for chlorate or oxygen formation is found at pH 7.1. These results highlight the need to consider a complex mixture of different Cl species when addressing the chemistry of hypochlorous acid containing solutions.
Hypochlorous acid
Hypochlorous acid is a chlorine oxoacid with formula HOCl; a weak, unstable acid, it is the active form of chlorine in water. It has a role as a human metabolite, an EC 3.1.1.7 (acetylcholinesterase) inhibitor and an EC 2.5.1.18 (glutathione transferase) inhibitor. It is a member of reactive oxygen species and a chlorine oxoacid. It is a conjugate acid of a hypochlorite.
ChEBI
The hypochlorite ion is ClO-. A hypochlorite compound is a chemical compound containing this group. Hypochlorite is a strong oxidant. About 28% of oxygen consumed by phagocytes upon activation participates in its generation. Hypochlorite reacts with H2O2 producing singlet oxygen (1O2) - a strong initiator of Lipid peroxidation. O-sub 2, along with H2O2 can serve as the substrate for myeloperoxidase. When this takes place, Hypochlorite is formed. Hypochlorite is able to modify antioxidants incorporated into lipoproteins such as Alpha-tocopherol, Beta-carotene, lycopene, and ubiquinol-10. It is also able to modify a number of proteins which possess antioxidant functions such as ceruloplasmin, transferrin, superoxide dismutase, and catalase. Active myeloperoxidase is found at sites of atherosclerotic damage to the arterial vessel wall in humans. Lipid peroxidation is known to contribute to the development of pathological processes, among them atherosclerosis. The present hypothesis is that blood lipoproteins modified by Lipid peroxidation play a key role in the pathogenesis of this disease. One of the possible reasons for the appearance of oxidized blood lipoproteins in blood is the interaction of native blood lipoproteins with the reactive oxygen species generated by stimulated neutrophils, monocytes and other cells. The main reaction of Hypochlorite with unsaturated lipid is probably the generation of chlorohydrins. However, this reaction is not accompanied by generation of free radicals and Lipid peroxidation. This reaction is accompanied by the production of free radicals (but not singlet oxygen), probably alkoxyl radicals, which may play a role in the initiation of Hypochlorite-induced Lipid peroxidation. (PMID: 9260000, Biofactors.1997, 6(2):181-90. ).
Hypochlorous acid
Chloric(I) acid
IUPAC namechloric(I) acid
Other nameshydrogen hypochlorite
hydrogen chlorate(I)
hypochlorous acid
Identifiers
CAS number7790-92-3
EINECS number232-232-5
SMILESHOCl
Properties
Molecular formulaHClO
Molar mass52.46 g/mol
Appearancecolorless aqueous solns
Density ? g/cm3, ?
Melting point(? K)
Boiling point°C (? K)
Solubility in watersoluble
Acidity (pKa)7.497[1]
Hazards
Main hazardsoxidizer
Related Compounds
Related compoundsCl2
Ca(OCl)2NaOCl
Except where noted otherwise, data are given for
materials in their standard state
(at 25 °C, 100 kPa)
Infobox disclaimer and references
Chloric(I) acid is a weak acid with the chemical formula HOCl. It forms when chlorine dissolves in water. It cannot be isolated in pure form due to rapid equilibration with its precursor (see below). HOCl is used as a bleach, an oxidizer, a deodorant, and a disinfectant.
Additional recommended knowledge (Pictures)
How to Quickly Check Pipettes?
Weighing the Right Way
12 Practical Tips for Correct Weight Handling
Formation
Addition of chlorine to water gives both chloric(I) acid and hydrochloric acid[2] (HCl):
Cl2 + H2O → HOCl + HCl
Uses
In organic synthesis, HOCl converts alkenes to chlorohydrins.[3]
In biology, hypochlorous acid is generated in activated neutrophils by myeloperoxidase mediated peroxidation of chloride ions, and contributes to the destruction of bacteria.[4][5][6]
Hypochlorous acid is the active sanitizer in hypochlorite based swimming pool products.
Chemical reactions
In aqueous solution, hypochlorous acid partially dissociates into the anion hypochlorite ClO-:
HOCl OCl- + H+
Salts of hypochlorous acid are also called hypochlorites. One of the best known hypochlorites is NaClO, the active ingredient in bleach.
In the presence of sunlight, hypochlorous acid decomposes into hydrochloric acid and oxygen, so this reaction is sometimes seen as:
2Cl2 + 2H2O → 4HCl + O2
HOCl is considered to be a stronger oxidant than chlorine.
Reactivity of HOCl with biomolecules
Hypochlorous acid reacts with a wide variety of biomolecules including DNA, RNA,[6][7][8][9] fatty acid groups, cholesterol[10][11][12][13][14][15][16][17] and proteins.[2][18][13][19][20][21][22]
Reaction with protein sulfhydral groups
Knox et al.[20] first noted that HOCl was a sulfhydral inhibitor that in sufficient quantity could completely inactivate proteins containing sulfhydral groups. This is because, HOCl oxidises sulfhydral groups leading to the formation of disulfide bonds[23] that can result in crosslinking of proteins. The HOCl mechanism of sulfhydral oxidation is similar to that of chloramine, and may only be bacteriostatic, because once the residual chlorine is dissipated, some sulfhydral function can be restored.[19] One sulfhydral containing amino acid can scavenge up to four molecules of HOCl.[22] Consistent with this, it has been proposed that sulfhydral groups of sulfur containing amino acids can be oxidized a total of three times by three HOCl molecules, with the fourth reacting with the α-amino group. The first reaction yields sulfenic acid (R-SOH) then sulfinic acid (R-SO2H) and finally R-SO3H. Each of those intermediates can also condense with another sulfhydral group causing cross linking and aggregation of proteins. Sulfinic acid and R-SO3H derivatives are only produced at high molar excesses of HOCl, and disulfides are primarily formed at bacteriocidal levels.[9] Disulfide bonds can also be oxidized by HOCl to sulfinic acid.[23] Because the oxidation of sulfhydrals and disulfides evolves hydrochloric acid,[9] this process results in the depletion HOCl.
Reaction with protein amino groups
Hypochlorous acid reacts readily with amino acids that have amino group side chains, with the chlorine from HOCl displacing a hydrogen resulting in an organic chloramine.[24] Chlorinated amino acids rapidly decompose but protein chloramines are longer lived and retain some oxidative capacity.[22][5] Thomas et al.[5] concluded from their results that most organic chloramines decayed by internal rearrangement and that fewer available NH2 groups promoted attack on the peptide bond resulting in cleavage of the protein. McKenna and Davies[25] found that 10 mM or greater HOCl was necessary to fragment proteins in vivo. Consistent with these results it was later proposed that the chloramine undergoes a molecular rearrangement releasing HCl and ammonia to form an amide.[26] The amide group can further react with another amino group to form a Schiff base causing cross linking and aggregation of proteins.[13]
Reaction with DNA and Nucleotides
Hypochlourous acid reacts slowly with DNA and RNA as well as all nucleotides in vitro.[7][27] GMP is the most reactive because HOCl reacts with both the heterocyclic NH group and the amino group. Similarly TMP with only a heterocyclic NH group that is reactive with HOCl is the second most reactive. AMP and CMP which only have a slowly reactive amino group are less reactive with HOCl.[27] UMP has been reported to be reactive only at a very slow rate.[6][7] The heterocyclic NH groups are more reactive than amino groups and their secondary chloramines are able to donate the chlorine.[9] These reactions likely interfere with DNA base pairing and consistent with this, Prütz[27] has reported a decrease in viscosity of DNA exposed to HOCl similar to that seen with heat denaturation. The sugar moieties are unreactive and the DNA backbone is not broken.[27] NADH can react with chlorinated TMP and UMP as well as HOCl. This reaction can regenerate UMP and TMP and results in the 5-hydroxy derivative of NADH. The reaction with TMP or UMP is slowly reversible to regenerate HOCl. A second slower reaction that results in cleavage of the pyridine ring occurs when excess HOCl is present. NAD+ is inert to HOCl.[27][9]
Reaction with lipids
Hypochlorous acid reacts with unsaturated bonds in lipids, but not saturated bonds, and the OCl− ion does not participate in this reaction. This reaction occurs by hydrolysis with addition of chlorine to one of the carbons and a hydroxyl to the other. The resulting compound is a chlorhydrin.[10] The polar chlorine disrupts lipid bilayers and could increase permeability.[11] When chlorhydrin formation occurs in lipid bilayers of red blood cells, increased permeability occurs. Disruption could occur if enough chlorhydrin is formed.[10][16] The addition of preformed chlorhydrins to red blood cells can affect permeability as well.[12] Cholesterol chlorhydrins have also been observed,[11][14] but do not greatly affect permeability, and it is believed that Cl2 is responsible for this reaction.[14]
Mode of disinfectant action
Escherichia coli exposed to hypochlorous acid lose viability in less than 100 ms due to inactivation of many vital systems.[2][28][29][30][31] Hypochlorous acid has a reported LD50 of 0.0104 ppm - 0.156 ppm[32] and 2.6 ppm caused 100% growth inhibition in 5 minutes.[25] However it should be noted that the concentration required for bactericidal activity is also highly dependent on bacterial concentration.[20]
Inhibition of glucose oxidation
In 1948, Knox et al.[20] proposed the idea that inhibition of glucose oxidation was a major factor in the bacteriocidal nature of chlorine solutions. He proposed that the active agent or agents diffused across the cytoplasmic membrane to inactivate key sulfhydral containing enzymes in the glycolytic pathway. This group was also the first to note that chlorine solutions (HOCl) inhibited sulfhydral enzymes. Later studies have shown that at bacteriocidal levels, the cytosol components do not react with HOCl.[1] In agreement with this, McFeters and Camper[33] found that aldolase, an enzyme that Knox et al.[20] proposes would be inactivated, was unaffected by HOCl in vivo. It has been further shown that loss of sulfhydrals does not correlate with inactivation.[19] That leaves the question what causes inhibition of glucose oxidation. The discovery that HOCl blocks induction of β-galactosidase by added lactose[34] led to a possible answer to this question. The uptake of radiolabeled substrates by both ATP hydrolysis and proton co-transport may be blocked by exposure to HOCl preceding loss of viability.[1] From this observation it proposed that HOCl blocks uptake of nutrients by inactivating transport proteins.[1][18][35][33] The question of loss of glucose oxidation has been further explored in terms of loss of respiration. Venkobachar et al.[36] found that succinic dehydrogenase was inhibited in vitro by HOCl and this led to the investigation of the possibility that disruption of electron transport could be the cause of bacterial inactivation. Albrich et al.[6] subsequently found that HOCl destroys cytochromes and iron-sulfur clusters and observed that oxygen uptake is abolished by HOCl and adenine nucleotides are lost. Also observed was, that irreversible oxidation of cytochromes paralleled the loss of respiratory activity. One way of addressing the loss of oxygen uptake was by studying the effects of HOCl on succinate dependent electron transport.[37] Rosen et al.[31] found that levels of reductable cytochromes in HOCl treated cells were normal, and these cells were unable to reduce them. Succinate dehydrogenase was also inhibited by HOCl, stopping the flow of electrons to oxygen. Later studies[29] revealed that Ubiquinol oxidase activity ceases first, and the still active cytochromes reduce the remaining quinone. The cytochromes then pass the electrons to oxygen, which explains why the cytochromes cannot be reoxidized as observed by Rosen et al.[31] However, this line of inquiry was ended when Albrich et al.[2] found that cellular inactivation precedes loss of respiration by using a flow mixing system that allowed evaluation of viability on much smaller time scales. This group found that cells capable of respiring could not divide after exposure to HOCl.
Depletion of adenine nucleotides
Having eliminated loss of respiration Albrich et al.[2] proposes that the cause of death may be due to metabolic dysfunction caused by depletion of adenine nucleotides. Barrette et al.[34] studied the loss of adenine nucleotides by studying the energy charge of HOCl exposed cells and found that cells exposed to HOCl were unable to step up their energy charge after addition of nutrients. The conclusion was that exposed cells have lost the ability to regulate their adenylate pool, based on the fact that metabolite uptake was only 45% deficient after exposure to HOCl and the observation that HOCl causes intracellular ATP hydrolysis. Also confirmed was; that at bacteriocidal levels of HOCl, cytosolic components are unaffected. So it was proposed that modification of some membrane bound protein results in extensive ATP hydrolysis, and this, coupled with the cells inability to remove AMP from the cytosol depresses metabolic function. One protein involved in loss of ability to regenerate ATP has been found to be ATP synthetase.[18] Much of this research on respiration reconfirms the observation that relevant bacteriocidal reactions take place at the cell membrane.[34][18][38]
Inhibition of DNA replication
Recently it has been proposed that bacterial inactivation by HOCl is the result of inhibition of DNA replication. When bacteria are exposed to HOCl there is a precipitous decline in DNA synthesis that precedes inhibition of protein synthesis, and closely parallels loss of viability.[25][39] During bacterial genome replication, the origin of replication (oriC in E. coli) binds to proteins that are associated with the cell membrane, and it was observed that HOCl treatment decreases the affinity of extracted membranes for oriC, and this decreased affinity also parallels loss of viability. A study by Rosen et al[40] compared the rate of HOCl inhibition of DNA replication of plasmids with different replication origins and found that certain plasmids exhibited a delay in the inhibition of replication when compared to plasmids containing oriC. Rosen’s group proposed that inactivation of membrane proteins involved in DNA replication are the mechanism of action of HOCl.
Safety
HOCl is a strong oxidizer and can form explosive mixtures.
Categories: Hypochlorites | Acids | Disinfectants
This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Hypochlorous_acid". A list of authors is available in Wikipedia.